Introduction
Clinical decision support (CDS) technology has the potential to improve health outcomes by offering physicians an informational resource to support review and application of best pratices.1 The Multiple Myeloma Research Foundation (MMRF) and Intermountain Healthcare (IMH) conducted a study to assess the suitability of a single health system's data for a myeloma-specific CDS tool that visualizes treatment pathways, and to assess the effort needed to support a CDS program.2 This research is part of a longer-term effort to explore how CDS technology can help:
- increase awareness of and apply treatment guidelines by visualizing pathways for specific MM patient cohorts
- improve understanding of treatment variation within health systems
- improve outcomes research by showing relationships between treatments and outcomes
Methods
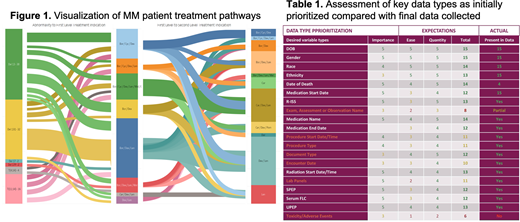
IA12 data from the CoMMpass study3 was used to create a CDS tool prototype. These data were aggregated into state and transition maps to identify nodes and pathways with corresponding outcomes, including response, progression-free survival (PFS), and overall survival (OS). Intervening patient states were displayed using Sankey diagrams [Fig. 1].
We also tested if EMR data from a community health system (i.e., IMH) could support such visualization. The team designed a study protocol and obtained IRB approval. Inclusion criteria included patients with active MM between January 2016-June 2018; adult aged 18 years to 89 years at diagnosis of active or smoldering MM.
An IMH-specific data dictionary was assessed for variable importance, quantity, and ease of acquisition. [Table 1]. IMH then manually abstracted prioritized structured (eg: labs) and non-structured (eg: notes) data for use in the tool.
Results
Ninety-six of an initial 146 patients meeting eligibility criteria had sufficient data usable for the study, reflecting 44 unique drug combinations across 9 lines of therapy. The tool was able to associate and visualize all patients and their clinical states and transitions to their outcomes. Clinical data was typically complete (99% of the time), including key clinician-derived data, such as ECOG scores (78%) and treatment response (99%). 569 person-hours were required to conduct the abstraction activity on 96 cases, averaging 5.9 hours/patient
Discussion
The IMH portion of the study supports the hypothesis that a community health system can provide sufficient high-quality information to power a CDS tool with priority features. Only 65% (96/146) of the initial study group had usable data because some patients had received partial care outside of the IMH integrated delivery network (IDN) leaving associated data inaccessible. Initial biostatistical analysis suggests that roughly 750-1000 complete patient records would be required for statistically significant outcomes research with granularly stratified cohorts.
The MMRF is currently recruiting 5-7 additional large IDNs to obtain the patients to power more generalizable functionality.
References
1 McKie PM, Kor DJ, Cook DA, Kessler ME, Carter RE, Wilson PM, et al. Computerized advisory decision support for cardiovascular diseases in primary care: a cluster randomized trial. Am J Med [Internet]. 2019 Dec 18 [cited 2020 Mar 5]. Available from: https://doi.org/10.1016/j.amjmed.2019.10.039
2 Garcelon N, Burgun A, Salomon R, Neuraz A. Electronic health records for the diagnosis of rare diseases. Kidney Int [Internet]. 2020 Jan 14 [cited 2020 Mar 5]. Available from: https://doi.org/10.1016/ j.kint.2019.11.037
3 Christofferson A, Nasser S, Aldrich J, Penaherrera D, Legendre C, Benard B, et al. Integrative analysis of the genomic landscape underlying multiple myeloma at diagnosis: an Mmrf Commpass analysis. Blood. 2017 Dec 7; 130 (Supplement 1): 326.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal